Copper
|
|
| Copper
was known to some of the oldest civilizations on record, and
has a history of use that is at least 10,000 years old. Most
copper is obtained from sulphide ores. These are found admixed
with large quantities of gangue and the initial content of
copper may be very low. A concentrate containing 15 to 35%
copper is produced by flotation. |
|
|
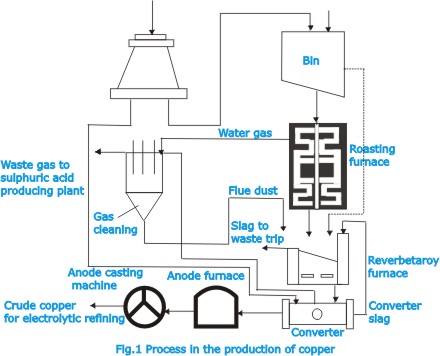
For
the purpose of eliminating some of the sulphur and certain
impurities, the concentrate is usually roasted before smelting.
Roasting is carried out in multiple-hearth furnaces, in which
oxidizing reactions take place: sulphur is eliminated as the
dioxide and metallic sulphides i.e. iron and some copper remain
behind as oxides.
|
|
 |
|
| Copper
is essential for computers to work. Copper is used in building
the integrated circuits, chips, and the printed circuit boards
of the computer alone. Copper is becoming more and more popular
to use in the layers of the build-up of a chip. IBM announced
a plan to use copper in its computer chip rather than aluminum.
Doing so would make the computer to be cheaper and would allow
it to make faster calculations. And one more thing statue
of liberty is made of copper. It is green because the copper
has combined with carbon dioxide and water in the air. |
|
| Copper,
as native copper, is one of the few metals to naturally occur
as an uncompounded mineral. No one knows exactly when copper
was first discovered, but earliest estimates place this event
around 9000 BC in the Middle East. A copper pendant was found
in what is now northern Iraq that dates to 8700 BC. It is
probable that gold and iron were the only metals used by humans
before copper. |
|
 |
|
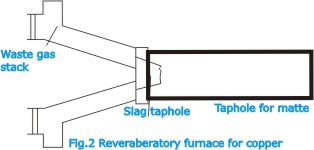
| The
mixture calcine contains the sulphides of iron and copper,
together with gangue material and impurities. The next step
consists in producing a molten artificial sulphide of copper
and iron, known as matte, containing all the copper and desired
amount of iron. For producing the matte smelting operation
is generally carried out in a reverberatory furnace (Fig.2)
fired with oil, natural gas or pulverized coal. |
|
 |
|
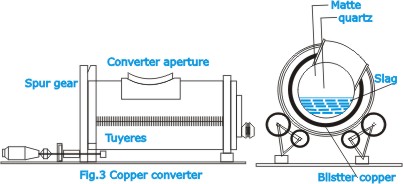
| The
charge is fed through the roof, and molten material collect
in a pool at the bottom. Slag, which rises to the top, is
tapped off. The matte collects at the bottom of the pool and
is discharged through a taphole. The molten matte is fed to
a converter (Fig.3) in which the iron and sulphur are removed
by oxidation, which is effected by blowing air through the
molten mass and is based on the fact that copper has a lower
affinity for oxygen than has iron or sulphur.
|
|
| The
reactions in the converter occur in several stages. First
the iron oxidizes and forms a slag with silica, which has
been added to the charge; this slag is tapped off, the copper
then being present as the sulphide. Further oxidation results
in the formation of metallic copper with a small amount of
copper oxide and other impurities. The converter in which
the process is performed is a large revolving refractory-lined
drum. |
|
| |
|
| The
copper obtained in the converter is subjected to further refining
treatment, which consist in fire refining (in furnaces) followed
by electrolytic refining. Fire refining is done in small reverberatory
furnaces or in revolving furnaces similar to the copper converter.
Air is blown through the molten material to oxidize all impurities;
the oxides rise to the surface and are skimmed off. This is
then followed by a reduction process which is performed by
forcing the ends of green logs into the molten metal to form
highly reducing gases. |
|
The
copper obtained as a result of this treatment is called tough
pitch. For further refining, it is cast into anodes for electrolytic
refining cells (Fig.4). The system most widely used is known
as the multiple system, comprising separate anodes and cathodes;
the latter consist of thin sheets of high-purity copper. When
an electric current is passed through cells, copper is dissolved
from the anodes and is deposited in a very pure form on the
cathodes. When these have grown to a thickness of about ½
inch they are replaced by fresh starting sheets. |
|
Ore
treatment may, alternatively, be carried out by hydrometallurgical
processes in which the ore is treated with a solvent that
dissolves the copper and leaves the undesirable material unaffected.
This principle is applied more particularly to the oxide ores
of copper, or to sulphide ores after suitable roasting, sulphuric
acid being used as the leaching solvent. Elaborate washing,
filtration and purification of the leach solution are associated
treatments.
|
|
| Copper
is essential in all plants and animals. Copper is carried
mostly in the bloodstream on a plasma protein called ceruloplasmin.
When copper is first absorbed in the gut it is transported
to the liver bound to albumin. Copper is found in a variety
of enzymes, including the copper centers of cytochrome c oxidase
and the enzyme superoxide dismutase (containing copper and
zinc). In addition to its enzymatic roles, copper is used
for biological electron transport. |
|
|
o
DISCLAIMER o
CONTACT US |