|
|
|
Lead |
|
| The
lead ore most commonly mined in galena, which is the sulphide
of lead (PbS). It forms intimately mixed with other metalliferous
minerals, such as sphalerite (zinc sulphide), cooper pyrites
and iron pyrites. The ore has to be concentrated, e.g., by
flotation, in order to separate the galena from the sphalerite
and other minerals that may be present. |
|
| Lead
has been commonly used for thousands of years because it is
widespread, easy to extract and easy to work with. It is highly
malleable and ductile as well as easy to smelt. Metallic lead
beads have been found in Çatalhöyük dating
back to 6400 B.C. In the early Bronze Age, lead was used with
antimony and arse. |
|
| Lead
also refers collectively to the organic and inorganic compounds
of lead, which are toxic. Lead poisoning was documented in
ancient Rome, Greece, and China. In the 20th century, the
use of lead in paint pigments was sharply reduced because
of the danger of lead poisoning, especially to children. By
the mid-1980s, a significant shift in lead end-use patterns
had taken place. Much of this shift was a result of the U.S.
lead consumers' compliance with environmental regulations
that significantly reduced or eliminated the use of lead in
non-battery products, including gasoline, paints, solders,
and water systems. |
|
 |
|
| Lead
use is being further curtailed by the European Union's RoHS
directive. Lead may still be found in harmful quantities in
stoneware, vinyl such as that used for tubing and the insulation
of electrical cords, and brass manufactured in China. Between
2006 and 2007 many children's' toys made in China were recalled,
primarily due to lead in paint used to color the product. |
|
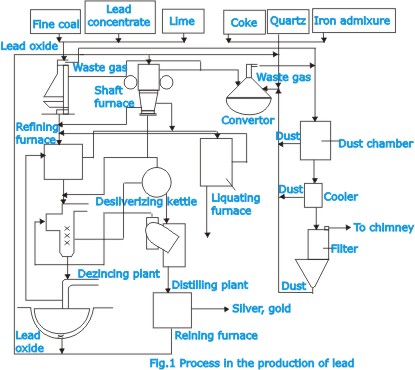
| Subsequent
treatment of the concentrate thus obtained consists in roasting
followed by reduction in a vertical-shaft furnace, a form
of blast furnace. Roasting is basically performed by heating
the lead ores, blended with suitable fluxing minerals, on
a traveling endless grate through which air is sucked. In
this way the material is sintered converted into lumps called
sinter, which are then mixed with coke and charged into the
shaft furnace (Fig.2). Air is forced into the furnace at the
bottom. |
|
|
The
coke, which uses as fuel and reducing agent, reacts with the
sinter to reduce the oxides and yield liquid lead, which is,
however, contaminated with other metals like silver, copper,
zinc, tin, antimony, bismuth, arsenic, etc. The non reduced
components form a liquid slag which floats on the liquid metal.
Preparing the charge and operating the furnace call for great
skill. In particular, the charge must contain the correct
proportions of iron, lime and silica to produce a liquid slag
that can readily be separated from the metal; it is also essential
to maintain the proper balance of coke and sinter.
|
|
| |
|
| When
the impure liquid lead (bullion) cools, some of the impurities,
especially copper, separate out as drosses, which are further
processed to extract the copper. Further removal of the copper
may be effected by treatment of the bullion with sulphur.
Antimony, tin and arsenic are removed by elective oxidation
in a reverberatory furnace or by treatment of the bullion
with chemical reagents to separate out these metals in the
form of salt-type compounds. |
|
| On
cooling, the zinc forms a dross or crust which contains nearly
all the silver and other metallic impurities. The dross is
skimmed off, and the silver is recovered from it in a separate
process. The zinc is distilled off and used over and over
again (Fig.3). After desilverizing, the lead may have to be
debismuthized, which is done by a process somewhat like desilverizing
but using calcium and magnesium instead of zinc to form dross
with the bismuth. |
|
|
An
alternative method of treating the impure bullion is by electrolytic
refining. The bullion is cast into plates which serve as anodes
in electrolytic tanks. The electric current causes the lead
at the anode to dissolve, and pure lead is deposited at the
cathode. All these refining processes can produce pig lead
of very high purity (99.999%). |
|
|
o
DISCLAIMER o
CONTACT US |
|
|
|