Nitric Acid |
|
|
For
a long time all nitric acid (HNO3) used to be produced from
nitrates occurring in nature. For example, nitric acid can
be obtained by adding sulphuric acid to saltpeter (potassium
nitrate), whereby the latter undergoes decomposition.
|
|
| In
the pure state the acid is a colorless liquid which boils
at 87 oC. The boiling point rises with increasing dilution.
As pure nitric acid will always undergo some decomposition
when left to stand, especially when exposed to the action
of light, acid with a concentration exceeding 90% almost invariably
contains some dissolved NO2. This decomposition process can
be stopped by diluting the acid with water. |
|
 |
|
| Nitric
acid produces a very convincing aged effect on maple and especially
on pine. It is an extremely dangerous chemical to work with
full-strength, so basically recommend 10-20% solution. After
applying the nitric acid, heat the wood with an electric heat
gun to develop the color effect. Then neutralize the nitric
acid with a solution of baking soda dissolved in water. Allow
at least one week drying time before finishing.
|
|
 |
|
| The
various processes for the commercial manufacture of nitric
acid are based on any of three principles: the decomposition
of nitrates more particularly Chile saltpeter with sulphuric
acid: the direct synthesis of NO from nitrogen and oxygen
in an electric arc, followed by the last two reactions of
the ammonia process or the catalytic oxidation of ammonia.
|
|
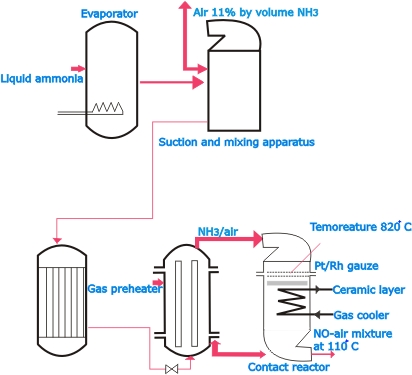
| The
ammonia process is now most widely employed, more particularly
with platinum as the catalyst. In certain variants of the
process the catalyst may, however, be Fe2O3, Mn2O3 or Bi2O3.
In this process liquid ammonia is vaporized in an evaporator
and is mixed with air. The gas mixture (10% ammonia and 90%
air) makes its way through a filter and a preheater to the
contact reactor, in which it passes over platinum gauze (the
catalyst) heated initially to about 900 oC. Heat liberated
in the reaction maintains the temperature of the catalyst.
In the reactor about 90% of the ammonia is oxidized to nitric
oxide: 4NH3 + 5O2 -> 4 NO +6H2O
|
|
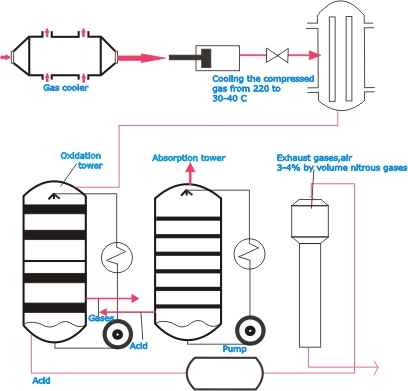
| Directly
after the platinum gauze are filtering agents which trap the
unstable platinum compounds present in the gas discharged
from the reactor: the platinum precipitated in this way is
recovered. This gas, of which 97 ½% is NO, is cooled
in the gas cooler. On leaving the cooler, it is passed through
absorption towers, filled with rings made of ceramic material,
in which two reactions take place. In the first tower the
nitric oxide is oxidized to nitrogen dioxide. 2 NO+O2 ->
2 NO2. In the following towers (four in all) the dioxide reacts
with water to form nitric acid: 3 NO2 +H2O -> 2 HNO3.
|
|
| |
|
| The
requisite water is added in the last tower. In the preceding
towers the NO2 is brought into contact not with water, but
with nitric acid solution, which is circulated by pumps and
passed through cooling apparatus to remove the heat evolved
in the reaction, as low temperatures are favorable to the
absorption reactions. In a degasifying tower air is blown
through the acid to remove such amounts of NO gas as are still
present in it. The exhaust gas from the final absorption tower
contains 0.3 to 0.4 % NO.
|
|
| The
installation described here produces nitric acid in a concentration
of between 40 and 60%. A higher concentration (Upto 99.5%)
is obtainable by distillation with concentrated sulphuric
acid. Nitric acid is used in the manufacture of fertilizers,
explosives, lacquers, dyes, plastics and synthetic fibers.
|
|
Alone,
it is useful in metallurgy and refining as it reacts with
most metals, and in organic syntheses. When mixed with hydrochloric
acid, nitric acid forms Aqua Regia, one of the few reagents
capable of dissolving gold and platinum. |
|
| Nitric
acid is a powerful oxidizing agent, and the reactions of nitric
acid with compounds such as cyanides, carbides, and metallic
powders can be explosive. Reactions of nitric acid with many
organic compounds, such as turpentine, are violent and hypergolic
(i.e., self-igniting). Concentrated nitric acid dyes human
skin yellow due to a reaction with the keratin. These yellow
stains turn orange when neutralized. |
|
|
o
DISCLAIMER o
CONTACT US |