Phosphorus |
|
|
Phosphorus
was discovered by German alchemist Hennig Brand in 1669 through
a preparation from urine, which contains considerable quantities
of dissolved phosphates from normal metabolism. Working in
Hamburg, Brand attempted to create the infamous Philosopher's
stone through the distillation of some salts by evaporating
urine, and in the process produced a white material that glowed
in the dark and burned brilliantly. Since that time, phosphorescence
has been used to describe substances that shine in the dark
without burning.
|
|
| Phosphorus
was recognized as a chemical element at the emergence of the
atomic theory that gradually occurred in the late part of
the 18th century and the early 19th century, and was formulated
by John Dalton.
|
|
| Phosphorus
was first made commercially, for the match industry, in the
19th century, by distilling off phosphorus vapor from precipitated
phosphates heated in a retort. The precipitated phosphates
were made from ground-up bones that had been de-greased and
treated with strong acids. This process became obsolete in
the late 1890s when the electric arc furnace was adapted to
reduce phosphate rock.
|
|
 |
|
 |
|
| Phosphorus
occurs in nature only in the form of the salts of phosphoric
acid. From these it is obtained by reduction. There are three
allotropic forms of elementary phosphorus: white, red and
black. Of these, black phosphorus is the form that is most
stable at room temperature; it is obtained from the white
form by the application of high pressures.
|
|
| Black
phosphorus is mainly of scientific interest, whereas the other
two allotropic forms are technically important. White phosphorus
melts at 44.1°C, when finely divided, reacts with atmospheric
oxygen even at room temperature. Red phosphorus is obtained
from white phosphorus by heating the latter in a closed container.
|
|
| Phosphorus
is prepared by heating calcium phosphate with carbon and silica
(SiO2) in an electric furnace. The reaction is represented
by the following equation:
2Ca3(PO4)2 + 6SiO2 +10 C -> 6Ca SiO4+P4+10 CO
|
|
| |
|
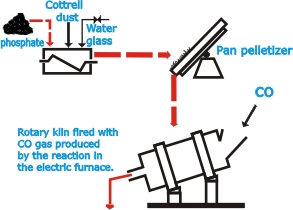
| The
phosphate is fed to the furnace in lump form. To make them
suitable for processing in this way, finely granular phosphates
first have to be agglomerated by palletizing. Agents used
for binding the particles together into pellets are soda water
glass (sodium silicate), Cottrell dust obtained from electrostatic
precipitation processes and other admixtures, which are intimately
mixed with the phosphate grains in a screw mixer and then
palletized in a revolving pan. The pellets are transformed
to firm, hard balls (nodules) by sintering at high temperatures
in rotary kilns or on special sintering grates. The nodules,
mixed with coke and silica pebbles, are fed to the furnace.
|
|
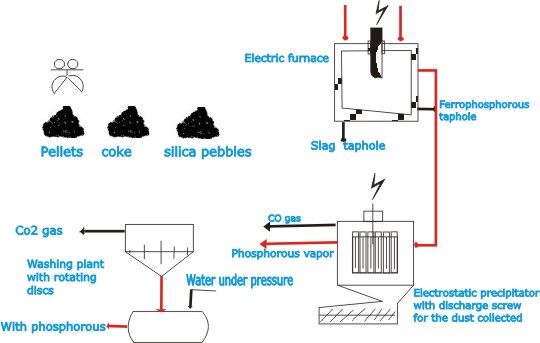
| The
three-phase electric furnace consists of a steel tank of which
the bottom part is lined with hard-burned carbon blocks and
the top part with fireclay bricks. At the bottom are two tapholes
for tapping the ferrophosphorus and the slag respectively.
The furnace cover is provided with openings for the three
electrodes, the feed pipe and the gas outlet. The electrodes
are made of carbon and are fed from above at a rate sufficient
to compensate for loss by burning. |
|
| The
gas discharged from the furnace, consisting of phosphorus
and CO, is passed through Cottrell-type electro-static dust
precipitators (dust filters) in which the dust in the gas
is trapped and collected. These precipitators are heated to
prevent condensation of phosphorus inside them. The dust is
returned to the sintering plant. |
|
| The
exit gases, which have a temperature of 250° –350°
C, are passed to Stroder washers in which the phosphorus is
condensed. The white phosphorus obtained in this process is
stored under water to prevent the spontaneous combustion that
results from contact with air. The CO gas that is discharged
from the washers is utilized for heating the sintering plant
and steam boilers or is burned at the top of high flare stacks.
|
|
| Phosphorus
is used in the manufacture of detergents. The plastics industry
uses phosphorus-based plasticizers. Red phosphorus is employed
in the friction striking surfaces on match boxes. |
|
|
o
DISCLAIMER o
CONTACT US |